|
|||||||||||
|
Electrophoresis |
|||||||||||
|
Datenschutzerklärung >> |
|||||||||||
|
|
|||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
EDC Electrophoresis Development & Consulting, Vor dem Kreuzberg 17, 72070 Tübingen (Germany) |
|
April 03, 2025 |
|
|
||||||||||||||||||||||||||||||||
 |
|
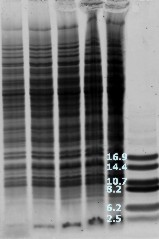
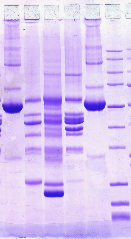
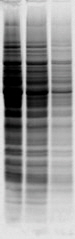
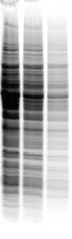
Basic peptides do not like SDS |