 |
|||||||
|
Electrophoresis |
|||||||
|
Datenschutzerklärung >> |
|||||||
|
|
|||||||
|
Classical SDS and 2-Dimensional Electrophoresis |
|||||||||||||||||||||
|
Due to a multi-phase buffer-syste we have separation down to 1500 Dalton in a normal 15% gel even without a gradient. |
|||||||||||||||||||||
|
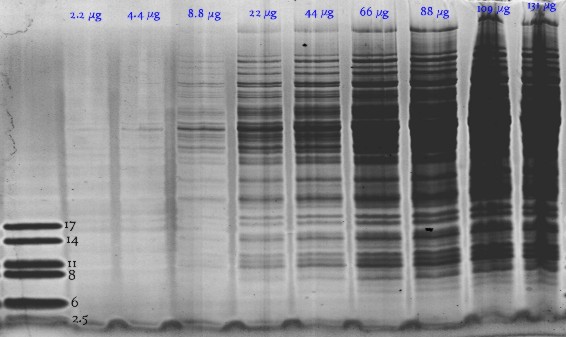
Bacillus subtilis in 1-dimensional SDS-electrophoresis: SDSGel 15% 25S. Hot Coomassie stain |
|||||||||||||||||||||
 |
|||||||||||||||||||||
|
K.Buettner, Greifswald (Germany) |
|||||||||||||||||||||
|
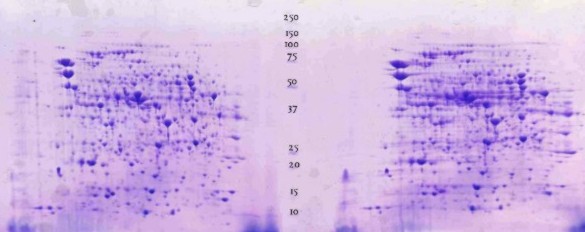
E.coli in classical 2D electrophoresis: |
|||||||||||||||||||||
 |
|||||||||||||||||||||
|
Bacillus subtilis run on IPG pH 4-7, then on a horizontal gel 15%T: The smallest peptide visible is ~10 kD |
|||||||||||||||||||||
 |
|||||||||||||||||||||
|
K.Buettner, Greifswald (Germany) |
|||||||||||||||||||||
|
EDC Electrophoresis Development & Consulting, Vor dem Kreuzberg 17, 72070 Tübingen (Germany) |
|||||||||||||||||||||