|
Stain preparation.
Coomassie brilliant blue G250 (xylene brilliant cyanin G) was purchased from K and K Laboratories, Inc. (Catalog No. 27551).
To a 0.2% (w/v) aqueous solution of the dye was added an equal volume
of 2 N H2SO4.
After the solution was mixed well, it was allowed to stand for at least 3 hr. The resulting precipitate was removed by gravity filtration through Whatman No.1 paper.
The volume of the clear brown filtrate was measured and one-ninth volume of 10 N KOH was added, producing a dark purple solution.
To the dark purple solution, 100% (w/v) trichloroacetic acid was added to a final concentration of 12% (w/v).
The resulting clear light blue solution was ready for use.
The staining solution can be stored for several months without loss of
effectiveness. It may be reused several times, but the pH of the solution
must be maintained below 1.0.
Staining procedure.
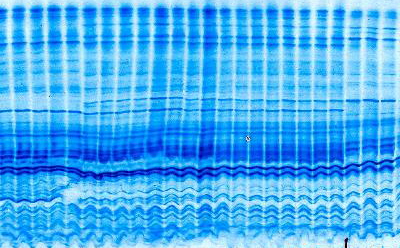
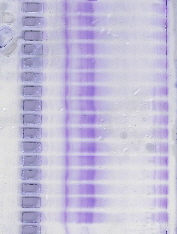
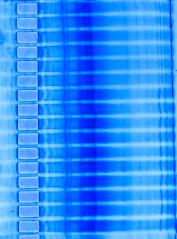
After electrophoresis, the polyacrylamide gel was added directly to the staining solution. For example, a 0.7 x 10 cm
cylindrical gel was placed in a test tube with 10-15 ml of stain.
Within 30 min, bands containing 5-10 ug of protein were evident. Color development for maximum sensitivity was achieved in 5-8 hr.
Gels could be stored in the staining solution without having the gel backgrounds stain significantly. If the gels were stored in water (10 -15 ml), there was a marked color intensification of the stained protein bands and gel background reduction producing optimal sensitivity. However, if the gels were placed in solutions containing sulfuric acid, trichloroacetic acid, or acetic acid, there was a rapid loss of intensity of the stained protein bands
|