|
|||||||
|
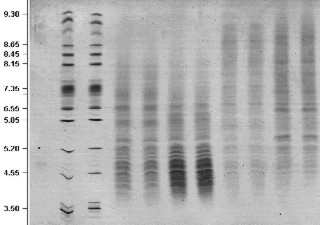
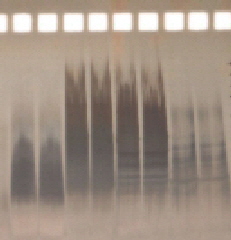
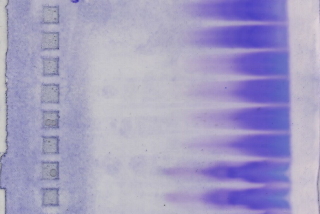
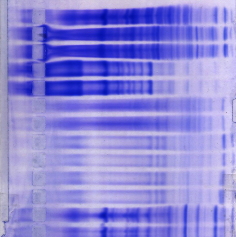
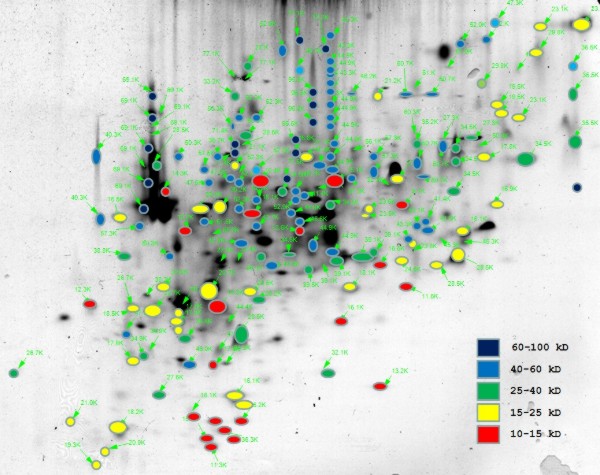
Electrophoresis |
|||||||
|
Datenschutzerklärung >> |
|||||||
|
|
|||||||
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
If SDS shows no good results as second dimension: |
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||
|
EDC Electrophoresis Development & Consulting, Vor dem Kreuzberg 17, 72070 Tübingen (Germany) |
|
- |