|
|||||||
|
Electrophoresis |
|||||||
|
Datenschutzerklńrung >> |
|||||||
|
|
|||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
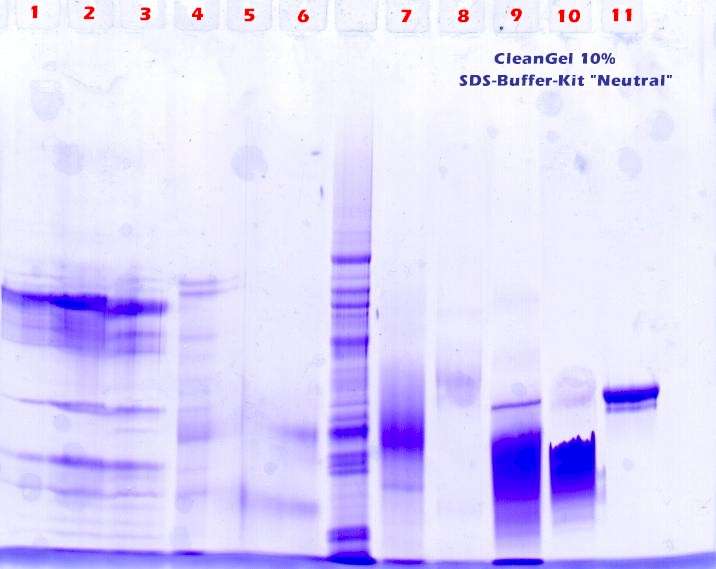
First trial: After the samples were filled in the sample-wells and the voltage is applied sample 1 – 5 jumped out of their slots and run over the stacking gel. This osmotic effect happens when there are too much ions in the sample volumes. It can also happen that 'salty samples' leave their slots even without an applied electric field. See figure 1. |
|
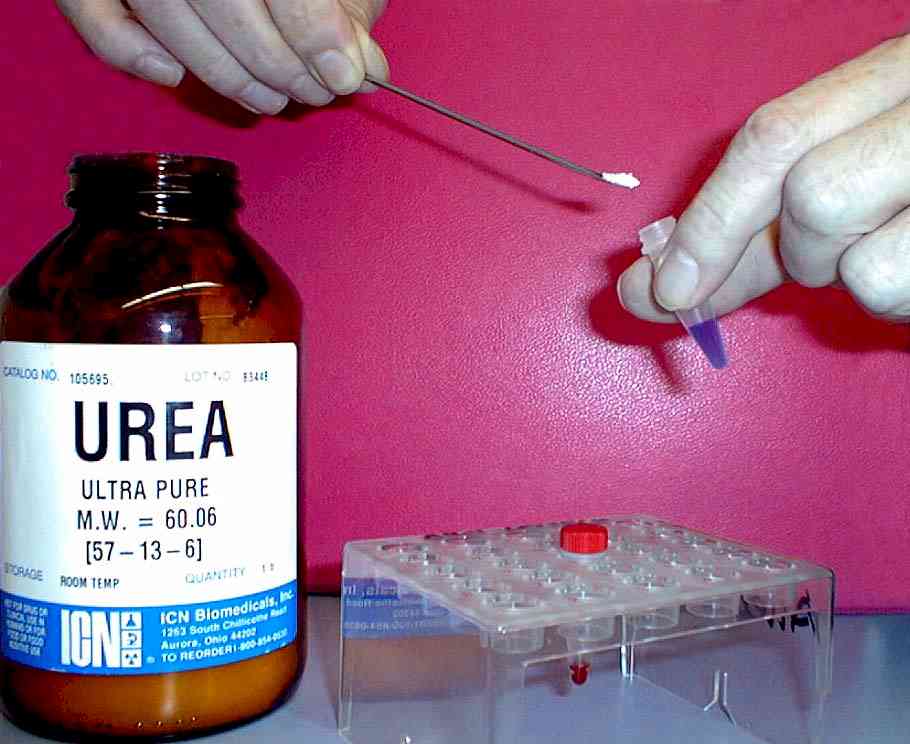
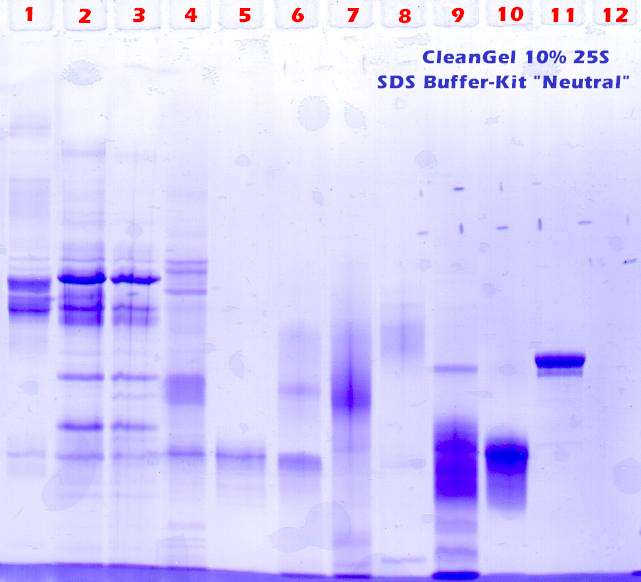
Second trial: To overcome this trouble, 1 spatula urea is added ( 3 – 4M) to 100 ul of the SDS-samples, see figure 3 . The effect of this procedure: Samples 1 – 5 stay in their slots. |
|
|
||||||||||||||||||||||||
|
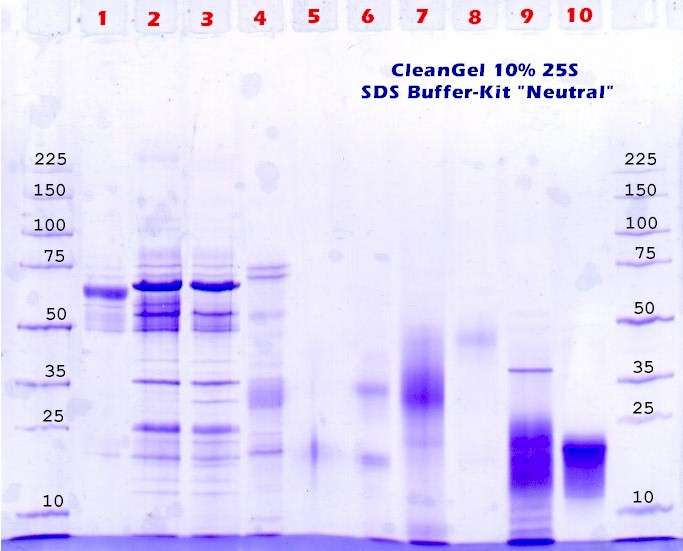
Special treatment for sample 5: This sample is passed through a special desalting column (NAP-5 GE 17-0853-01). This Sephadex G-25 containing ready-to-use column is especially designed to desalt 500ul DNA-samples. For proteins the procedure is changed a little: See table 1 and figure 5 and note that there is no sample dilution! |
|
|
||||||||||||||||||||||||||||||||||||||||||||||
|
|
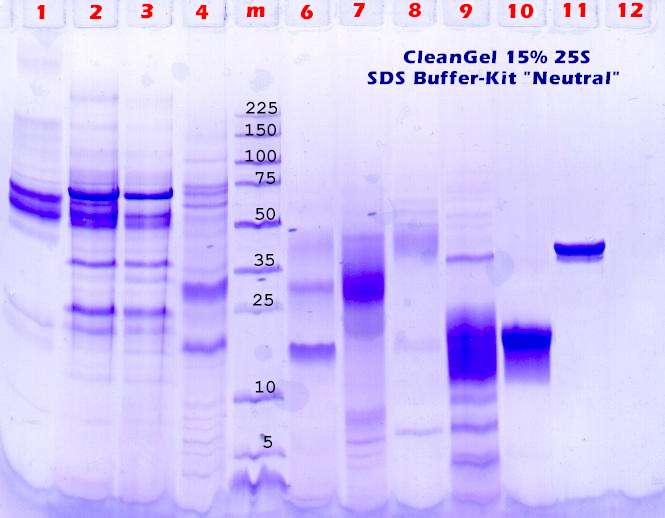
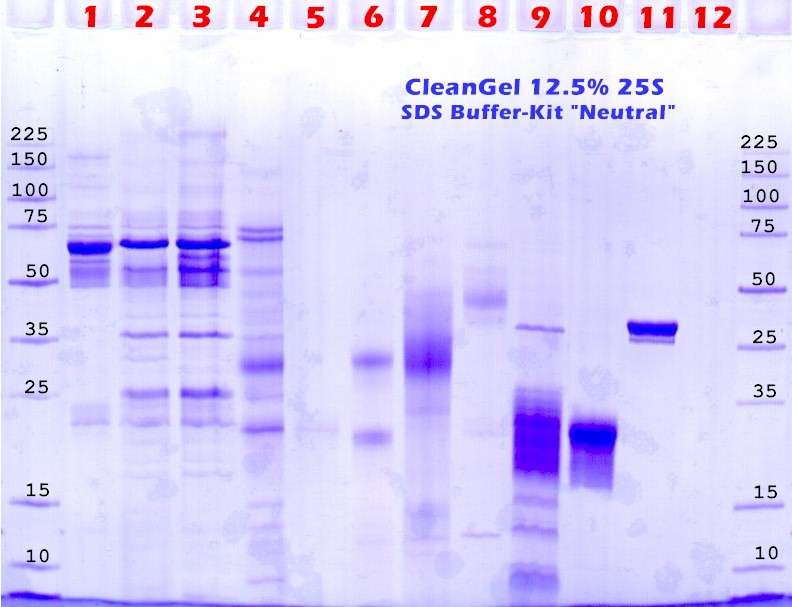
Last optimization: The gel concentration. |
|
|
||||||||||||||||||||||||||||
|
Remedy #2: Trichloroacetic Acid (TCA) -precipitation with optional sample-concentrating |
||||
|
Precipitate the proteins with 20% Trichloric Acid. Centrifuge samples. Wash 1 x with dist.water. Centrifuge. Resolubilize with sample buffer, + heating 3-5 min 95░. |
||||
|
Desalting (concentration) the samples |
||||
|
|
Remedy #3: Ethanol (EtOH) -precipitation and defatting with optional sample-concentrating |
|||
|
Desalting (concentration) the samples |
|||
|
|
|
|
EDC Electrophoresis Development & Consulting, Vor dem Kreuzberg 17, 72070 TŘbingen (Germany) |